2013/12/23
Excess S-adenosylmethionine reroutes phosphatidylethanolamine towards phosphatidylcholine and triglyceride synthesis.
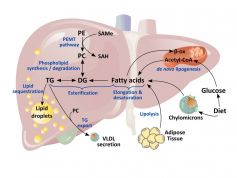
Nonalcoholic fatty liver disease (NAFLD) is the most common liver disease in Western countries, being frequently associated with obesity, dyslipidemia and insulin resistance, a group of disorders that constitute the metabolic syndrome. Although these conditions predispose the individual to develop NAFLD, our understanding of the mechanisms by which fat accumulates in the liver is not fully understood. S-adenosylmethionine (SAMe) has been widely described to be an essential regulator of liver lipid homeostasis. Moreover, in NAFLD patients, the levels of the Glycine N-methyltransferase (GNMT), the catabolic enzyme for SAMe metabolism are deeply decreased. In a joint effort between the Metabolomics Unit of CIC bioGUNE, the Department of Physiology, University of the Basque Country UPV/EHU, and the Keck School of Medicine, University of Southern California, the imbalance leading by a chronic excess of SAMe resulting in steatosis has been exhaustively investigated. Elevated SAMe in the mice model GNMT KO activates the synthesis of Phosphatidylcholine (PC) via Phosphatidylethanolamine (PE) methyltransferase (PEMT). In order to maintain a normal PC/PE ratio, the liver stimulates very low-density lipoprotein (VLDL) and high-density lipoprotein (HDL) export, and increases PC catabolism via phospholipase D or C, leading to increased diacylglycerol production. Importantly, as an adaptation to this increase of triglycerides and PC mobilization via VLDL export, increased SAMe or PC synthesis also stimulates fatty acid (FA) uptake and esterification, whereas de novo lipogenesis and FA β-oxidation remain unaffected. Significantly, when GNMT KO mice are placed on a methionine restricted diet, hepatic SAMe and PEMT flux is restored to normal levels, and liver lipids and VLDL secretion normalized. This cascade of events goes a long way towards explaining why a chronic imbalance in hepatic SAMe synthesis or catabolism is capable of inducing NAFLD.
This study has been published and highlighted in Hepatology.
Link to the article:
http://www.ncbi.nlm.nih.gov/pubmed/23505042
Highlighted in:
http://www.ncbi.nlm.nih.gov/pubmed/23703836
See a large version of the first picture





