2011/12/19
First Engineered Human GLTP
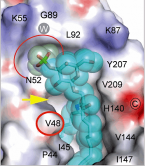
The rational engineering of proteins with desirable properties is an emerging field that relies on structural studies. A research team of CICbioGUNE leaded by Lucy Malinina reported first structure-guided engineering of the lipid binding features of human glycolipid transfer protein, GLTP [1]. Researchers found point mutations of the 'portal glycolipid entrance' in GLTP-fold that reversibly regulate lipid-chain access to the hydrophobic pocket and dramatically improve protein transfer-selectivity for sulfatide. The development of 'engineered GLTPs' with enhanced specificity for select glycolipids provides a potential new therapeutic approach for targeting lipid-mediated pathologies.
Reference:
(1) Samygina VR, Popov AN, Cabo-Bilbao A, Ochoa-Lizarralde B, Goni-de-Cerio F, Zhai X, Molotkovsky JG, Patel DJ, Brown RE, Malinina L. (2011) Enhanced Selectivity for Sulfatide by Engineered Human Glycolipid Transfer Protein. Structure. Nov 9; 19(11):1644-54
Figure:
Sulfatide accommodation in the sulfatide-selective GLTP mutant.
See a large version of the first picture





