2012/01/31
Integrative analysis of the ubiquitin proteome isolated using Tandem Ubiquitin Binding Entities (TUBEs)
According to a study carried out by researchers at CIC bioGUNE (Proteomics Unit and Proteomics Platform), the use of Tandem repeated Ubiquitin Binding Entities (TUBEs) under non-denaturing conditions followed by mass spectrometry analysis to study global ubiquitylation events may lead to the identification of potential drug targets as published in the Journal of Proteomics.
The research, published in the Journal of Proteomics, opens a door that could provide relevant information for basic research laboratories, clinicians and the pharmaceutical industry.
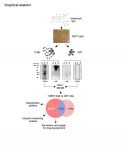
The successful use of proteasome inhibitors in clinical trials revealed the potential of the Ubiquitin Proteasome System for drug development. Protein remodeling through ubiquitylation is known to regulate the stability and activity of essential cellular factors through largely uncharacterized mechanisms. The use of Tandem repeated Ubiquitin Binding Entities (TUBEs) under non-denaturing conditions followed by mass spectrometry analysis to study global ubiquitylation events that may lead to the identification of potential drug targets. Using this approach we identified 643 proteins including known and unknown ubiquitin targets from human breast adenocarcinoma MCF7 cells treated with Adriamycin. Coherent with a global cellular response to this genotoxic insult, cellular factors identified are involved in protein synthesis, cellular transport, RNA post-transcriptional modification and signaling pathways regulating early stress responses. This includes components of large macromolecular complexes such as subunits and regulators of the proteasome, supporting the use of this method to characterize networks of molecular interactions coordinated by ubiquitylation. Besides, in vitro and in silico analysis confirmed that 84% of the total proteins identified here are ubiquitylated. In conclusion, this method represents an important step forward the identification of ubiquitin proteomes and ubiquitin-interacting proteins regulating cellular processes coordinated by protein ubiquitylation. Furthermore, the enrichment of potential biomarkers and already exploited drug targets, strongly suggest that this method could provide relevant information for basic research laboratories, clinicians and the pharmaceutical industry.
References
Lopitz-Otsoa F, Rodriguez-Suarez E, Aillet F, Casado-Vela J, Lang V, Matthiesen R, Elortza F, Rodriguez MS. Integrative analysis of the ubiquitin proteome isolated using Tandem Ubiquitin Binding Entities (TUBEs).
J Proteomics. 2011 Dec 10. [Epub ahead of print] PMID: 22178446 [PubMed - as supplied by publisher]
Science Direct Link
See a large version of the first picture





