2012/05/07
Post-transcriptional regulation of Schwann cell development
Schwann cells are the dominant glia cells in the peripheral nervous system (PNS) and associate with axons in peripheral nerve trunks. These cells control neuronal survival in the embryo, provide myelin that is essential for normal movement and sensation in the adult, and control regeneration and repair in injured nerves. The generation of myelinating Schwann cells from their progenitors and the maintenance of myelin in adult nerves are highly regulated processes and in both peripheral neuropathies and the Neurofibromatoses (two major classes of Schwann cell diseases), there are widespread alterations in gene expression. Many mechanisms regulate gene expression. In addition to transcription, posttranscriptional mechanisms such as mRNA processing, splicing, editing, transport, stability and translation play an important role in the control of gene expression. mRNA stability is carried out by RNA-binding proteins (RBPs) and is estimated to control expression of about 5-10% of all human genes.
A study led by investigators from the Metabolomics Unit at CIC bioGUNE-CIBERehd, in collaboration with investigators from the Genomics Analysis Platform, UCL, Keck School of Medicine (University of Southern California) and National Institute on Aging-Intramural Research Program (NIH) have shown that the the RNA-Binding Protein Human Antigen R (HuR) controls global changes in gene expression during Schwann cell development. In the article by Iruarrizaga-Lejarreta and colleagues published in the Journal of Neuroscience, the authors have found that HuR was highly expressed in immature Schwann cells, where genome-wide identification of its target mRNAs in vivo in mouse sciatic nerves using ribonomics showed an enrichment of functionally-related genes regulating these processes. HuR co-ordinately regulated expression of several genes to promote proliferation, apoptosis and morphogenesis in rat Schwann cells, in response to NRG1, TGFβ and laminins, three major signals implicated in this patterning event. Strikingly, HuR also binds to several mRNAs encoding myelination-related proteins, but contrary to its typical function, negatively regulated their expression, likely to prevent ectopic myelination during development. These functions of HuR correlated with its abundance and subcellular localisation, which were regulated by different signals in Schwann cells.
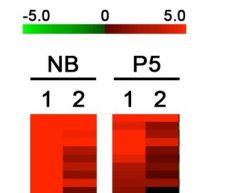
Figure: RIP-ChIP analysis of in vivo HuR mRNA targets in sciatic nerves from new-born and 5 days old mice.
See a large version of the first picture





