2009/12/11
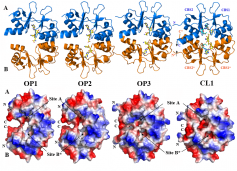
Protein MJ0100: an open-to-closed conformational change in its CBS motif pair.
Binding of 5'-methylthioadenosine and S-adenosyl-L-methionine to protein MJ0100 triggers an open-to-closed conformational change in its CBS motif pair.
The group of Alfonso Martínez from the Structural Biology Unit of CIC bioGUNE has recently published a study that helps to understand the molecular mechanisms mediated by Cystathionine beta-Synthase (CBS) domains upon binding of adenosine derivatives. The exact mechanism by which CBS domains act has remained opaque, but this work helps to put flesh on the bones of a dynamic model for allosteric regulation. The paper describes an extensive series of structures of the archaeal protein MJ0100 from Methanocaldococcus jannaschii with a variety of complexed ligands. Put together, the data revealed an open-to-closed transition which is important for regulating the activity of the N-terminal (and missing) domain of the protein and/or its binding to other proteins. Such open-to-closed transitions only have been observed previously in one other CBS domain-containing protein, the Mg2+ transporter MgtE (Hattori et al, Nature, 2007, 448, 1072-1075). The report has been published in "Journal of Molecular Biology" journal (Lucas et al, 2009) and is a result of a collaboration with University Miguel Hernández and University of the Basque Country.
See a large version of the first picture





