2015/08/31
The unexplored archaeal domain and its viruses
Archaeal viruses represents one of the most challenging and yet unknown area of investigation. Archaeal viruses live in extreme environments (alkaline, acidic conditions, hyper-salinity and elevated temperature), conditions reminiscent of what Earth would have looked alike billions of years ago.
Exploring this ecological niche sheds light on novel mechanisms of virus morphogenesis and biological functionalities. This knowledge might turn out useful in the future, in particular in biotechnological processes requiring non-standard operating conditions.
In the past structure-based approaches have been proven successful in revealing close relationships between viruses infecting organisms from different domains of life.
Here, using biochemical and cryo-electron microscopy techniques, we solved the structure of euryarchaeal, halophilic, internal membrane-containing Haloarcula hispanica icosahedral virus 2 (HHIV-2).
HHIV-2 was first isolated from the largest salt-pan in Europe (4.500 hectares), located at Margherita di Savoia (Foggia, Italy), in a virus sampling expedition carried out during summer 2007.

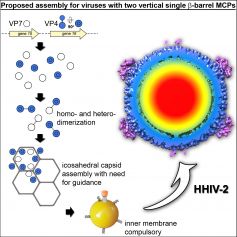
We show that the density of the two major capsid proteins (MCPs) recapitulates vertical single β-barrel proteins and that disulfide bridges stabilize the capsid. Below, ordered density is visible close to the membrane and at the five-fold vertices underneath the host-interacting vertex complex underpinning membrane-protein interactions. The HHIV-2 structure exemplifies the division of conserved architectural elements of a virion, such as the capsid, from those that evolve rapidly due to selective environmental pressure such as host-recognizing structures.
We propose that in viruses with two vertical single β-barrel MCPs the vesicle is indispensable, and membrane-protein interactions serve as protein-railings for guiding the assembly.
This work is the result of a long-standing and fruitful collaboration between the Abrescia (CIC bioGUNE) and the Bamford (University of Helsinki - Center of Excellence in Virus Research, Finland) groups.
This study has been published in Structure.
http://www.sciencedirect.com/science/article/pii/S0969212615003214
See a large version of the first picture





