2017/10/24
Unraveling the mechanism of glycosyl transfer reactions
Glycosyltransferases (GTs) play essential roles in nature. GTs catalyze the transfer of a sugar moiety to a broad range of acceptor molecules, including mono-, oligo- and polysaccharides, proteins, lipids, and small organic molecules. As a consequence, GTs generate a tremendous amount of structural diversity in biological systems, which are particularly apparent in molecular recognition events, during key processes in cell-cell and host-pathogen interactions.
Glycosyl transfer can proceed with either inversion or retention of the anomeric configuration with respect to the reaction substrates and products. The reaction mechanism of inverting GTs is well established. However, the elucidation of the catalytic mechanism of retaining GTs is still a matter of strong debate. By analogy with glycoside hydrolases, a double displacement mechanism via the formation of a covalent glycosyl-enzyme intermediate was first suggested. Such a mechanism would involve an enzymatic nucleophile positioned within the active site on the β-face of the donor substrate in close proximity to the anomeric reaction center. However, in the absence of a residue near the reaction center that can act as a nucleophile to form the glycosyl-enzyme intermediate, an alternative mechanism known as the SNi ‘internal return’, also called the SNi-like mechanism, has been proposed.
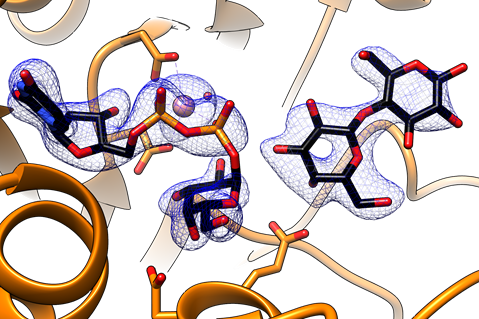
In this work we obtained two crystal structures of the 'retaining' α1,3-Galactosyltransferase (α3GalT) in the presence of its intact sugar donor UDP-Gal, the acceptor substrate lactose, and the divalent cation cofactor, without the use of any substrate derivative or protein mutant, at 2.1 and 2.2 Å resolution. The experimental structures reflect two snapshots of the reaction center in a productive mode for catalysis thereby providing for the first time the atomic coordinates of a native Michaelis complex for a GT containing a putative nucleophile in its active site. Our manuscript provides experimental support of a substrate-assisted SNi-type mechanism for α3GalT. Given that α3GalT, belongs to the only GT family for which a putative nucleophile has been described (GT6), these new experimental findings suggest the occurrence of a common substrate-assisted SNi-type mechanism for all retaining GTs.
See: Albesa-Jové D, Sainz-Polo MÁ, Marina A, Guerin ME. Structural Snapshots of
α-1,3-Galactosyltransferase with Native Substrates: Insight into the Catalytic
Mechanism of Retaining Glycosyltransferases. Angew Chem Int Ed Engl. 2017 Sep 28.
doi: 10.1002/anie.201707922.
http://onlinelibrary.wiley.com/doi/10.1002/anie.201707922/abstract
See a large version of the first picture
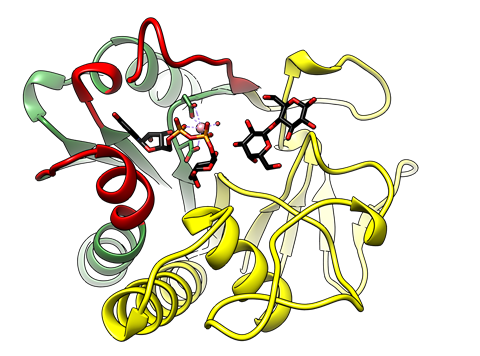
See a large version of the second picture