2010/04/21
Sorting of the Alzhermer's Disease Amyloid Precursor Protein Mediated by the AP-4 Complex.
A new Signal-Adaptor Interaction between Alzheimer's Disease Amyloid Precursor Protein and the AP-4 Complex has been published in the March edition of Developmental Cell, with Adriana L Rojas, manager of the macromolecular crystallography platform of CIC bioGUNE, as first co-author.
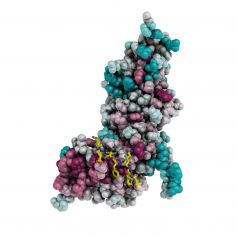
The paper reports the discovery of an entirely novel interaction between a signal from the cytosolic tail of the amyloid precursor protein (APP) and the µ4 subunit of the adaptor protein-4 (AP-4) complex. The novelty of this interaction lies not only in the properties of the APP signal but also in the nature of the binding site on µ4. Biochemical and X-ray crystallographic analysis reveals that the APP signal sequence and the location of the binding site on µ4 are distinct from those of other signal-adaptor interactions. In addition, disruption of this interaction leads to a shift in the distribution of APP from endosomes to the trans-Golgi network (TGN), and results in enhanced γ-secretase-catalyzed cleavage of APP to the pathogenic amyloid -β(αβ) peptide. AP-4 thus emerges as a novel regulator of APP trafficking and reveals a whole new mode of signal recognition by adaptor proteins.
See a large version of the first picture





