2011/05/19
Macromolecular crowding fails to fold a globular halophilic protein in cells
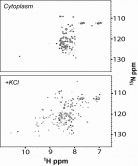
Scientists from the Structural Biology Unit in collaboration with researchers of the University of North Carolina at Chapel Hill have investigated the folding of a halophilic globular protein inside the cells by heteronuclear NMR spectroscopy. In vitro, the protein requires the presence of salt (NaCl or KCl) for a proper folding. Surprisingly, the protein fails to fold in the cellular cytoplasm of E. coli, revealing that the excluded volume effect, induced by the crowded milieu, is vastly compensated by non-specific interactions that preferentially stabilize the unfolded state. This result paves the way for the quantification of protein stability in vivo. The work has been recently published in the Journal of the American Chemical Society.
See a large version of the first picture