2011/07/01
Rapid identification of fluorochrome modification sites in proteins
Conjugation of a fluorescent dye to the ε-amino groups of protein lysine residues maximizes the detection sensitivity in binding assays or cell imaging. However the evaluation of the homogeneity of the population of the modified molecules and the identification of the modification sites is a time consuming process.
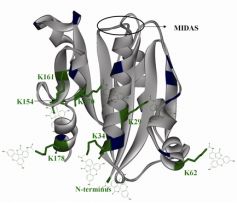
A collaborative work between the groups of Teruna J Siahaan (University of Kansas) and Francisco J Blanco (CIC bioGUNE) has set up a method for the rapid identification of the attachment sites of fluorescein molecules to the I-domain of the alfa chain of LFA-1 integrin. LFA-1 is involved in cell adhesion interactions and is a therapeutic target in cancer and autoimmune diseases. It binds to its ligands through the metal ion-dependent adhesion site (MIDAS) site of the I-domain.
The results show that one to seven fluorescein molecules were attached to the lysine residue(s) of the I-domain protein, with no modifications found in the MIDAS.
See a large version of the first picture