2011/12/12
Methionine adenosyltransferase 1A gene deletion disrupts hepatic VLDL assembly in mice
Very-low-density lipoprotein (VLDL) secretion provides a mechanism to export triglycerides (TG) from the liver to peripheral tissues, maintaining lipid homeostasis. In non-alcoholic fatty-liver disease (NAFLD) VLDL secretion disturbances are unclear. Researchers from the Faculty of Medicine, University of the Basque Country in collaboration with CIC-bioGUNE and the Keck School of Medicine at the University of Southern California, have found, as reported in the last issue of HEPATOLOGY, that methionine adenosyltransferase 1A (MAT1A) is required for normal VLDL assembly and plasma lipid homeostasis in mice and that impaired VLDL synthesis, mainly due to SAMe deficiency, contributes to NAFLD development in MAT1A-KO mice.
The role of MAT1A on VLDL assembly was investigated in two metabolic contexts: in 3-month-old MAT1A-knockout mice (3-KO), with no signs of liver injury, and in 8-month-old MAT1A-knockout mice (8-KO), harbouring non-alcoholic steatohepatitis (NASH). In 3-KO mice liver there is a potent effect of MAT1A deletion on lipid handling, decreasing mobilization of TG stores and secretion in VLDL and phosphatidylcholine synthesis via phosphatidylethanolamine N-methyltransferase. MAT1A deletion also increased VLDL-apoB secretion leading to small, lipid poor VLDL particles. Administration of SAMe to 3-KO mice for 7 days recovered crucial altered processes in VLDL assembly and features of the secreted lipoproteins. The unfolded-protein-response was activated in 8-KO mice liver, in which TG-accumulated and the phosphatidylcholine to phosphatidylethanolamine ratio reduced in the ER, whereas the secretion of TG and apoB in VLDL increased and the VLDL physical characteristics resembled that in 3-KO. MAT1A deletion also altered plasma lipid homeostasis, with an increase in lipid transport in LDL-subclasses and decrease in HDL-subclasses. The results are of high relevance for understanding the role of MAT1A on VLDL assembly and plasma lipid homeostasis.
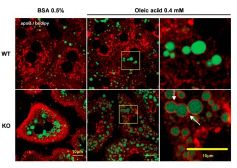
Figure. Increased VLDL-apoB secretion in 3-month-old MAT1A-KO mice is linked to altered localization of apoB in hepatocytes but not to autophagy. For confocal laser immunofluorescence analysis of apoB localization in hepatocytes, primary 3-month-old wild-type (WT) and MAT1A-knockout (MAT1AKO) mice hepatocytes were incubated for 4 hours in coverslips in DMEM complemented with 0.5% fatty acid-free BSA or 0.4 mM oleic acid in 0.5% BSA. Hepatocytes were then washed with PBS and fixed with 3.7% formaldehyde. After permeabilization (5% Triton X-100), cells were blocked (10% fetal bovine serum in PBS) and double labeled with antiapoB antibody (red) and BODIPY 493/503 (green).
See a large version of the first picture





