2012/06/04
NK cells are the main contributors to the chronification of liver inflammation in the absence of GNMT
Glycine N-methyltransferase (GNMT) is the key enzyme that catabolizes S-adenosylmethionine (SAMe), the main methyl donor of the body that tightly controls cell growth, apoptosis and proliferation. GNMT is regulated during chronic liver disease. We previously described that patients with cirrhosis show attenuated GNTM expression, which is absent in hepatocellular carcinoma (HCC), pointing out the main relevance of GNMT in protecting the liver against chronic liver disease. Accordingly, GNMT deficient mice (GNMT-/-) show spontaneous progression of NASH; from steatosis to chronic inflammation, cirrhosis and HCC.
Liver steatosis is a benign condition that may have no further consequences for the patient. However, when liver is chronically inflamed, the disease progresses to cirrhosis and HCC almost irreversibly. The liver is considered as an immune organ as it is highly enriched with innate immune cells that play a main role orchestrating the body's host defense. The liver contains a high amount of NK/NKT cells, which are classically described as the tumor surveillance cells that eliminate transformed and virally infected cells through mechanisms involving TRAIL-mediated apoptosis. A better understanding of the molecular mechanisms leading the progression from steatosis to chronic hepatitis would allow us to develop therapeutic strategies to counteract NASH.
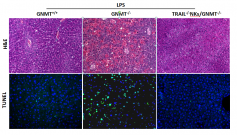
A study led by Dr. Naiara Beraza in the Metabolomics 2 laboratory directed by Dr. Martinez-Chantar at CIC bioGUNE-CIBERehd, has shown that NK cells are the main contributors to the chronification of liver inflammation in the absence of GNMT. In the article by Gomez-Santos and colleagues published in Hepatology, the authors have found that absence of GNMT in the liver promotes the activation of NK cells, triggering TRAIL-mediated cell death of GNMT-deficient hepatocytes. Moreover, GNMT-/- mice are highly susceptible to Concanavalin A-mediated fulminant hepatitis, a classical model of T-cell mediated injury. Interestingly, in GNMT-/- mice, ConA-fulminant hepatitis is mediated by NK but not NKT cells as adoptive transfer of TRAIL-deficient NK cells efficiently protects the liver from this insult. It is widely know that NASH progression is associated with intestine bacterial overgrowth and permeabilisation, which contributes to the aggravation of the liver disease. Interestingly, GNMT-/- mice are highly sensitive to endotoxin-liver damage. We found that NK cells are the main mediators of the detrimental effects of endotoxin in livers from GNMT-/- mice as specific depletion of NK cells impedes LPS-acute liver damage. Moreover, adoptive transfer of TRAIL-deficient NK cells efficiently protects the liver of GNMT-/- mice to LPS-acute injury, underscoring the main relevance of TRAIL-producing NK cells in mediating liver injury in the absence of GNMT.
Overall, the work of Gomez-Santos et al. point out that TRAIL-producing NK cells are the main mediators of the inflammatory response in the onset of NASH progression and suggests that therapeutic approaches based on NK cell inactivation may limit the chronification of the inflammatory response, a crucial condition for the progression of NASH.
See a large version of the first picture





