2013/05/16
Structural Basis for Rab1 De-AMPylation by the Legionella pneumophila Effector SidD
The covalent attachment of adenosine monophosphate (AMP) to proteins, a process called AMPylation (adenylylation), has recently emerged as a novel theme in microbial pathogenesis. While AMPylases from various pathogenic microorganisms have recently been characterized, the only virulence protein with de-AMPylation activity known to date is the Legionella pneumophila effector SidD which catalyzes AMP removal from the host GTPase Rab1. Thus, both AMPylation and de-AMPylation constitute a novel catalytic mechanism to precisely control the function and membrane dynamics of a host Rab GTPase. In spite of this pivotal role, the molecular mechanism of AMP removal and the structural determinants for Rab1 recognition by SidD have remained largely unexplored. The group of Dr. Aitor Hierro at CIC bioGUNE together with groups from the US National Institutes of Health (NIH) and the Barcelona Supercomputing Centre (BSC), have solved the crystal structure of the de-adenylylation domain of SidD and revealed the catalytic mechanism of Rab1 de-adenylylation. Surprisingly, the structure of SidD is not related to the other known enzyme with de-AMPylation activity, the Escherichia coli GS-ATase. Instead, the catalitic domain of SidD is remarkably similar to that of the metal-dependent protein phosphatases (PPMs), however with distinctive structural features to distinguish AMPylated Rab1 from similarly modified substrates. Importantly, this work provides a model for the SidD-Rab1 complex which sheds light into the specific details of substrate recognition and catalysis by this virulence factor.
Figure Caption:
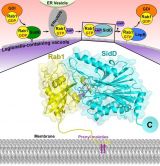
Structural model for the specific recognition of AMPylated Rab1 by SidD. Rab1 is anchored to the Legionella-containing vacuole membrane through its C-terminal hydrophobic prenyl tails whereas SidD is targeted to the same membrane via its C-terminal domain. Then through complementary shape, charge and hydrophobic interactions the N-terminal domain of SidD binds to AMPylated Rab1 and catalyzes the hydrolysis of the phosphodiester bond between AMP and Tyr77. The configuration of the complex shows how the prenylation anchor of Rab1 and the C-terminal targeting region of SidD are oriented towards the LCV membrane. Yellow, Rab1 in ribbon backbone representation with transparent surface; cyan, SidD in ribbon backbone representation with transparent surface; violet, Tyr77-AMP in stick representation; green spheres, Mg2+ ions; Lilac, prenyl groups.
Citation:
Chen Y, Tascón I, Neunuebel MR, Pallara C, Brady J, Kinch LN, Fernández-Recio J, Rojas AL, Machner MP and Hierro A. (2013) Structural Basis for Rab1 De-AMPylation by the Legionella pneumophila Effector SidD. PLoS Pathog 9(5): e1003382. doi:10.1371/journal.ppat.1003382
See a large version of the first picture





