2013/09/03
Structural Basis for the Interaction of the Golgi-Associated Retrograde Protein Complex with the t-SNARE Syntaxin 6.
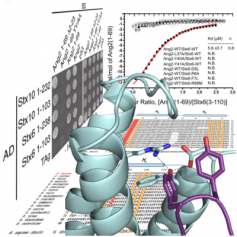
Fusion of transport vesicles with the appropriate target compartment is a tightly regulated process for cargo delivery in the endomembrane system of all eukaryotic cells. This process depends on tethering factors that capture transport vesicles and SNARE proteins that promote fusion of the vesicles with the target organelle. In addition to vesicle capture, tethering factors regulate SNARE function, but the mechanisms involved are not well understood. Now, the group of Dr. Aitor Hierro at CIC bioGUNE in collaboration with the group of Dr. Juan Bonifacino at the US National Institutes of Health (NIH), report the structural basis for the interaction of the tethering complex GARP with the SNAREs Syntaxin 6 and Syntaxin 10. X-ray crystallographic and binding analyses reveal a novel mode of interaction involving binding of a di-tyrosine motif near the N-terminus of the Ang2 subunit of GARP to a groove between helices a and b of the Syntaxin 6/10 N-terminal Habc domain. This interaction mode is different from that previously reported for the yeast Vps51 subunit of GARP and the yeast SNARE Tlg1. Importantly, the interaction is conserved throughout evolution, and also likely conserved for other tethering proteins and syntaxins.
The findings have been published in Structure:
http://www.cell.com/structure/retrieve/pii/S0969212613002499
See a large version of the first picture





