2015/12/09
Understanding proteins that only work with a pinch of salt
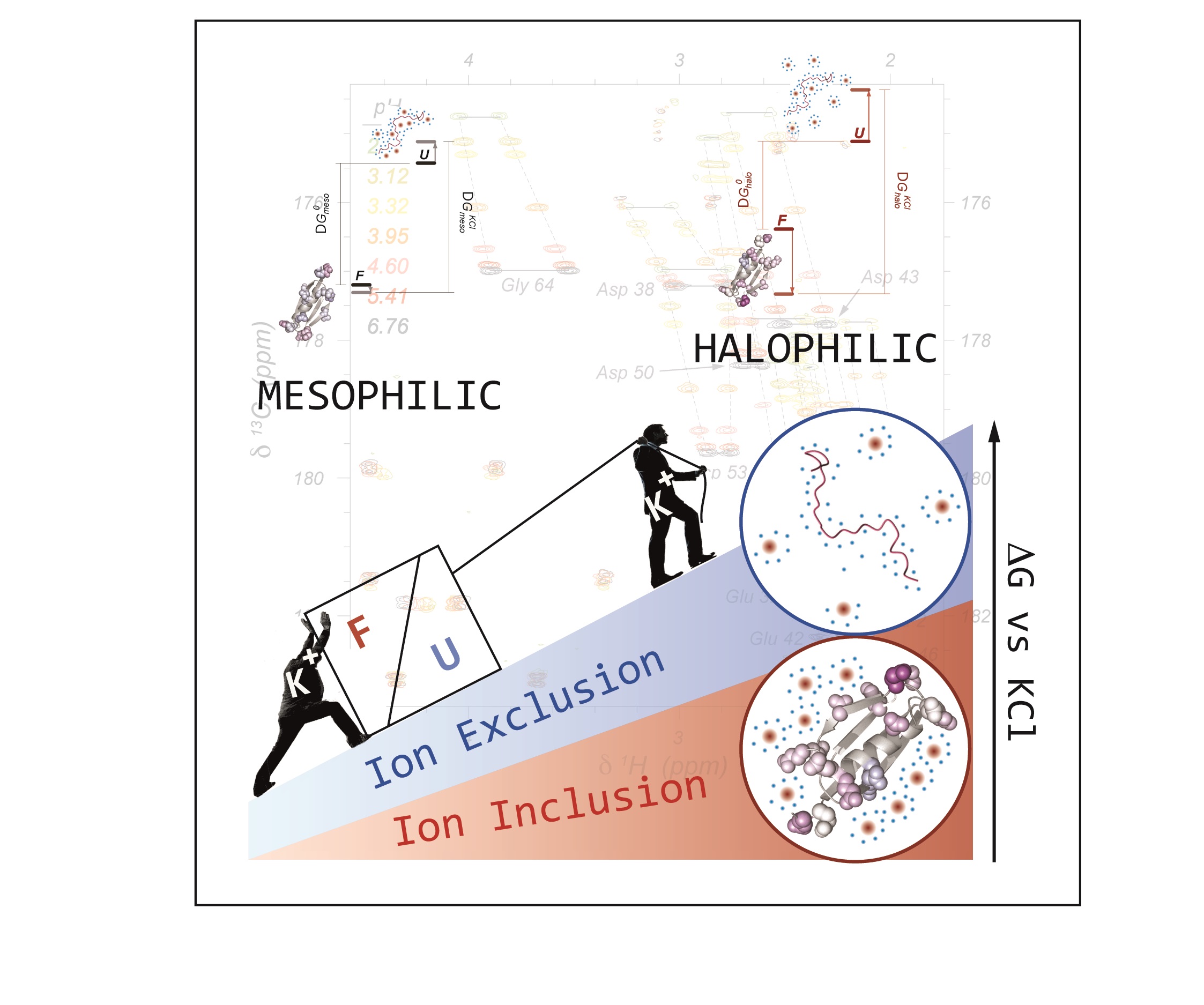
Life on earth exhibits an enormous adaptive capacity and living organisms can be found even in extreme environments. For instance, halophilic archaea thrieve in highly saline lakes with KCl concentrations between 2M and 6M. To avoid the osmotic shock, halophilic archea equalize the intracellular ionic strength to the environment and their proteome has been adapted to remain folded and fully functional. This is accomplished by a distinct amino acid composition where the negatively charged residues Asp and Glu are greatly enriched while Lys is depleted. In this contribution we propose and experimentally confirm a mechanism for haloadaptation through amplified synergistic ion effects that stabilise the folded and destabilise the unfolded state of halophilic proteins. To that end, we have emulated the evolutionary process of haloadaptation with natural and designed halophilic polypeptides and applied novel NMR methodology to study the different mechanisms contributing to protein haloadaptation at a per-residue level. Our analysis of an extensive set of NMR observables, determined over several proteins, allowed to disentangle synergistic contributions of protein haloadaptation: cation exclusion and electrostatic repulsion between negatively charged residues destabilize the denatured state ensemble while cumulative weak cation-protein interactions stabilise the folded conformations.
The work has been awarded with the front cover of the December issue of Chemistry & Biology.
See a large version of the first picture





